Synopsis of Research Projects and Academic Activities
Research focuses on thermal and nonthermal processing (ultraviolet light, ultrasound, chlorine dioxide gas, ozonated water, ozone, value-added plant extracts, and plant polyphenols) approaches to inactivate food-borne viruses (hepatitis A virus, human noroviruses and Aichi virus) and food-borne bacterial pathogens in foods and the food environment to prevent food-borne outbreaks and the spread of antimicrobial resistance, and to enhance food safety.
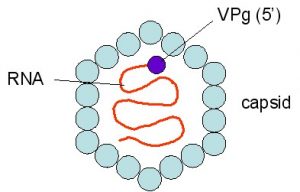
The role of plant-derived polyphenols in the prevention or alleviation of food-borne disease symptoms associated with food-borne viruses and the effect on the gut microbiome is another area of research interest.
Novel robust rapid methods for sample concentration and detection to track food-borne outbreaks and transmission of antimicrobial resistance, determine modes of transfer and pathogen persistence, and to apply appropriate control measures (including chemical sanitizers and hurdles) to decrease the risk of antimicrobial resistance development and the risk of foodborne illness
Teaching includes FDSC 521 Advanced Food Microbiology, (FDSC 501/601 Graduate Seminar, FDSC 421 Food Microbiology), FDSC 493 Undergraduate Research, FDSC 593 Graduate Research
Funding Sources & Industry Collaboration
USDA NIFA Enhancing Food Safety through Improved Processing Technologies-2 CAP projects,USDA NIFA Food Manufacturing Technologies, Southeastern Sun Grant Center, other USDA NIFA programs, and various Industry Collaborations to enhance food safety
Impact of Research
Foodborne bacterial and viral contamination in the food environment continues to result in human illness outbreaks. Novel rapid methods to sensitively detect these pathogens in the food environment and improved measures to control their spread are needed. Information on these methods remain lacking in part due to lack of suitable reproducible cell-culture methods to cultivate these viruses in the laboratory. The farmers, producers and the food industry remain in need of methods to help prevent food product recalls, and prevent significant losses to agriculture and protect public health.
Processing technologies such as thermal microwave technologies and non-thermal approaches using chlorine dioxide gas, ozonated water, ultrasound, ultraviolet light, photodynamic inactivation, and natural plant extracts are being researched in the laboratory in order to help processors to determine and apply appropriate parameters for bacterial and viral inactivation, to determine transmission routes and thus reduce the risk of outbreaks and spread of antimicrobial resistance. Novel field deployable sensitive detection assays and sampling and concentration methods are also being researched. These on-going research projects directly benefit the industry to inactivate pathogens and control transmission of disease outbreaks.
Results from this research continue to be presented at national and local scientific meetings including ASM, IFT, IAFP, and ISFEV and published in peer-reviewed scientific journals. Thus, the consumers and the food industry are provided with information on current technologies of benefit to human health and agriculture.
Graduate Students and
Andres Arreaza (MS): Process Engineer, Food Scientist, Kellogg’s
Sukriti Ailavadi (MS): Processing Supervisor, Sanderson Farms
Snigdha Sewlikar (MS): Product Development Manager, McKee Foods
Snehal Joshi (PhD): Senior Fellow, University of Washington
Malcond Valladares (PhD): Food Scientist Eurofins/Covance Foods
Lab Capabilities
Foodborne viruses continue to cause outbreaks and human illnesses worldwide. Our laboratory has unique capabilities for research to improve the identification, tracking and control of foodborne viruses of human health concern. Process validation of the inactivation of hepatitis A virus and human noroviruses requires the use of non-sporeforming, non-pathogenic bacterial surrogates that are being researched. Our laboratory has successfully collaborated with Washington State University on 2 USDA NIFA funded CAP projects (multidisciplinary) for the inactivation of foodborne viruses and process validation, that is on-going. In addition, our laboratory has begun collaboration with Tennessee State University on a USDA NIFA funded project to determine the dose requirement for foodborne viral inactivation in juices using ultraviolet light. We also have on-going USDA and Southeastern Sun Grant Center funded collaboration with a multidisciplinary group of scientists within UTIA (comprising of faculty members from Entomology and Plant Pathology; Forestry, Wildlife and Fisheries, and Animal Science-: on-going for >7 years) to utilize switchgrass extractives and related biomass as value-added products. We are also working with faculty from UT Electrical Engineering and Computer Science and UTIA Forestry, Wildlife and Fisheries, and Entomology and Plant Pathology to develop field deployable assays for caliciviruses and other infectious agents.
